Purpose
Dilutions are used in the clinical laboratory for a variety of purposes.
In chemistry, the most common use is when an analyte on a patient exceeds the analyzer’s analytical measuring range. Depending on the analyzer and lab protocol, the dilution may be made manually or by the analyzer itself (if at all).
In blood bank, serial dilutions may be performed for antibody titration. This helps determine the strength of an antibody.
In urinalysis, dilution may help when a urine has too many particulates to perform a microscopic quantitation.
Certain reagents may also require a dilution before use.
No matter what area of the lab you are in, you will likely have to do a dilution at some point.
Ratios
Ratios go hand in hand with dilutions. A ratio is the proportion of sample to diluent whereas a dilution is the proportion of sample to total volume.
Look at this graphic on the right for example. This is a 2:5 dilution with 2 parts sample in a 5 parts total. This means there would be 3 parts diluent (5 parts total – 2 parts sample), making this a 2:3 ratio.
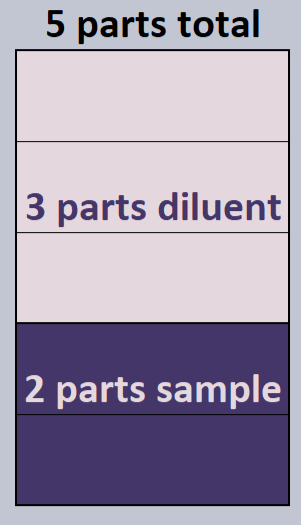
Knowing how to determine the ratio from the dilution and vice-versa is crucial. The ratio is usually most helpful when actually performing the dilution, as it tells me how much sample and diluent to add together.
Making Dilutions
Dilutions may be made with water, saline, or any other specified diluent. It is important to read any protocols or instructions to determine what diluent to use; never assume.
Once the proper diluent is selected, determine what dilution is needed. This is also usually specified by protocol or an instrument’s assay sheets. From there, the ratio of sample to diluent can be calculated. If the dilution is a 1:3, then it is a 1:2 ratio. In chemistry, this may be 250 uL of patient serum added to 500 uL saline for a total volume of 750 uL.
When a dilution is performed for testing, the result is multiplied by the dilution factor. The dilution factor is equal to the inverse of the dilution. In a 1:5 dilution, simply multiply the result by 5. If the dilution is more complicated, such as a 2:5 dilution, the dilution factor would be 5/2, or 2.5.
Serial Dilutions
Serial dilutions involve making dilutions on top of dilutions. This is especially useful in blood bank when determining antibody titers. In this case, it is usually a series of 1:2 dilutions, as shown below.
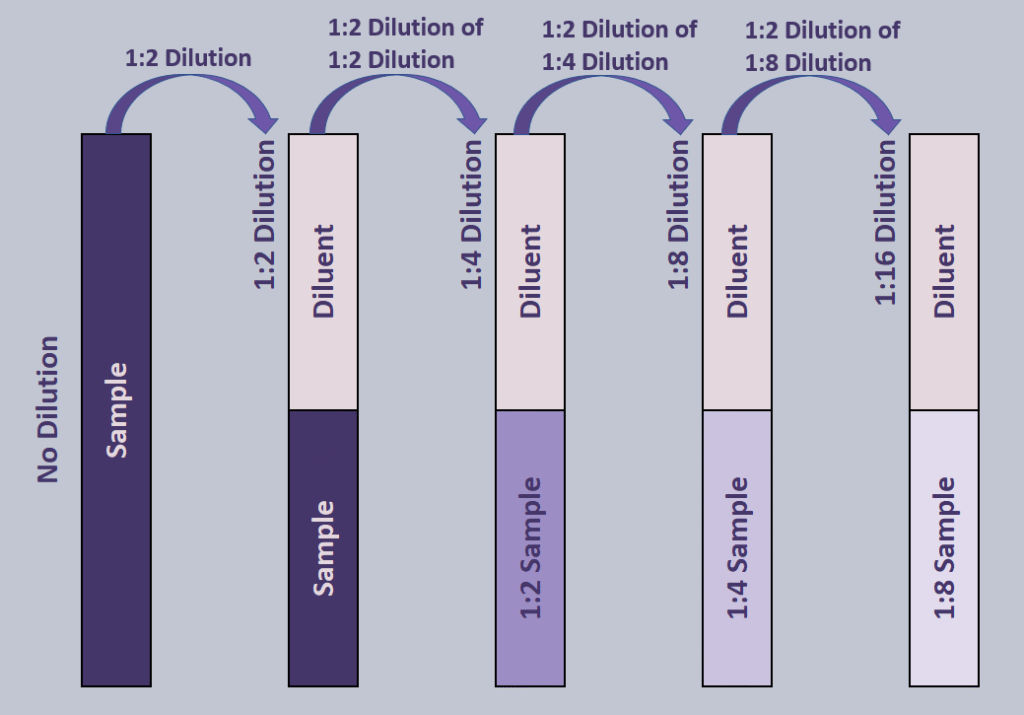
In a series of 1:2 dilutions, each dilution essentially halves the sample volume. A 1:2 dilution is made and homogenized. A subsequent 1:2 dilution is made on that solution, making it a total of a 1:4 dilution. To find the total dilution after performing a series of dilutions, simple multiply all the dilutions together. In this case it would be 1/2 * 1/2 * 1/2 * 1/2 * 1/2 (since 5 dilutions of 1:2 are made) or 1:16.
Not all serial dilutions will have the same dilution factor though. Look in the example below:
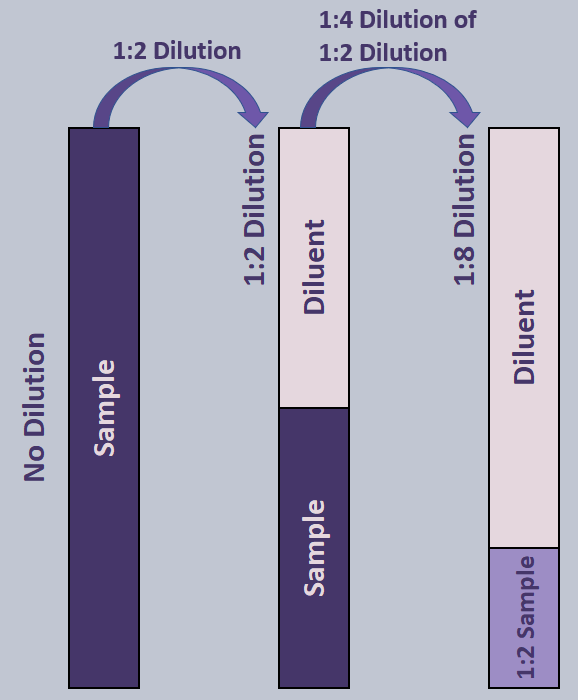
First, a 1:2 dilution is made, followed by a 1:4 dilution. To find the total dilution, still multiply the dilutions together: 1/2 * 1/4 = 1/8.